Can You Have a Half Degree of Unsaturation
A If you have too few number of degrees of unsaturation you must add degrees of unsaturation ie. Degrees of Unsaturation Formula.
Degrees Of Unsaturation Or Ihd Index Of Hydrogen Deficiency
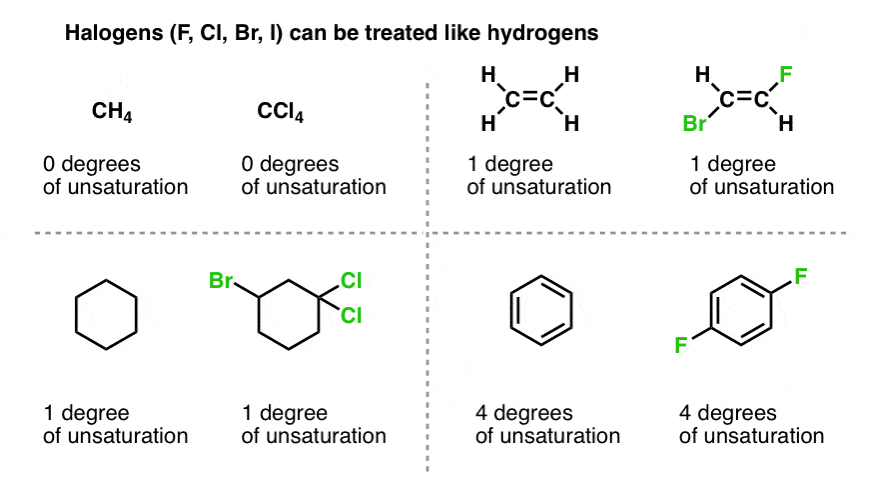
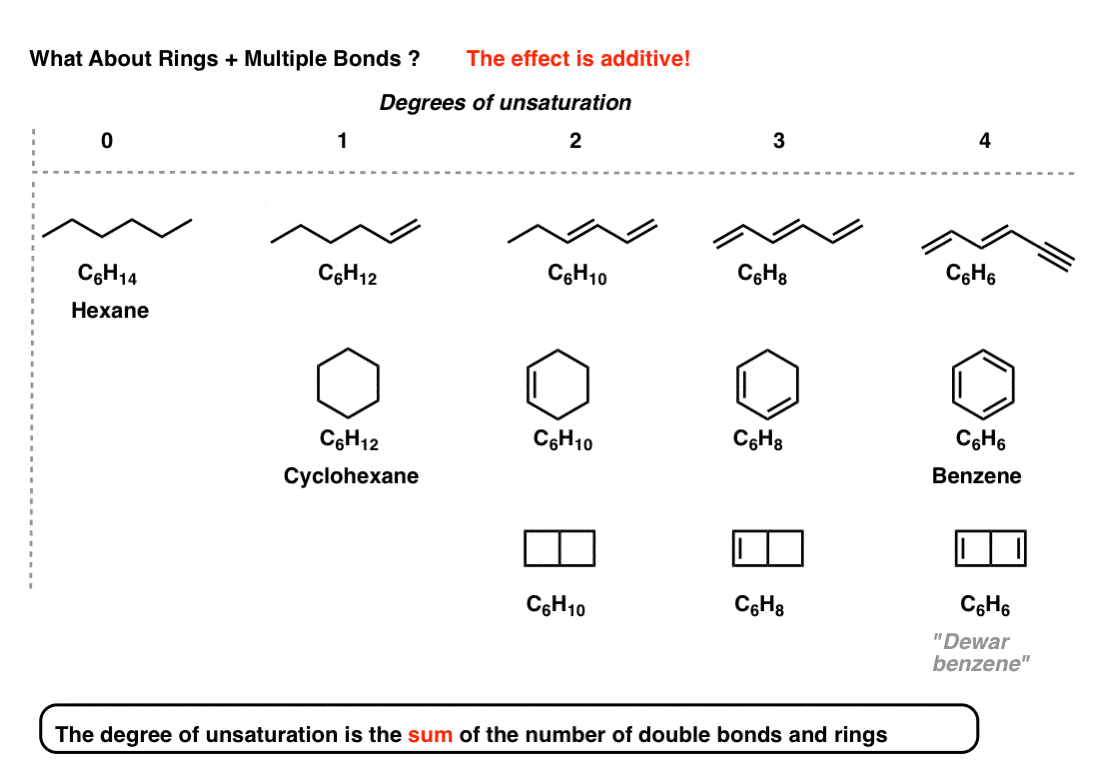
Benzene has 6 carbon atoms and 4 degrees of unsaturation 1 ring and 3 double bonds.
. Hence the degree of unsaturation formula divides the value by 2. If it was a single negative charge that would be half a degree of unsaturation. Hence the DoB formula divides by 2.
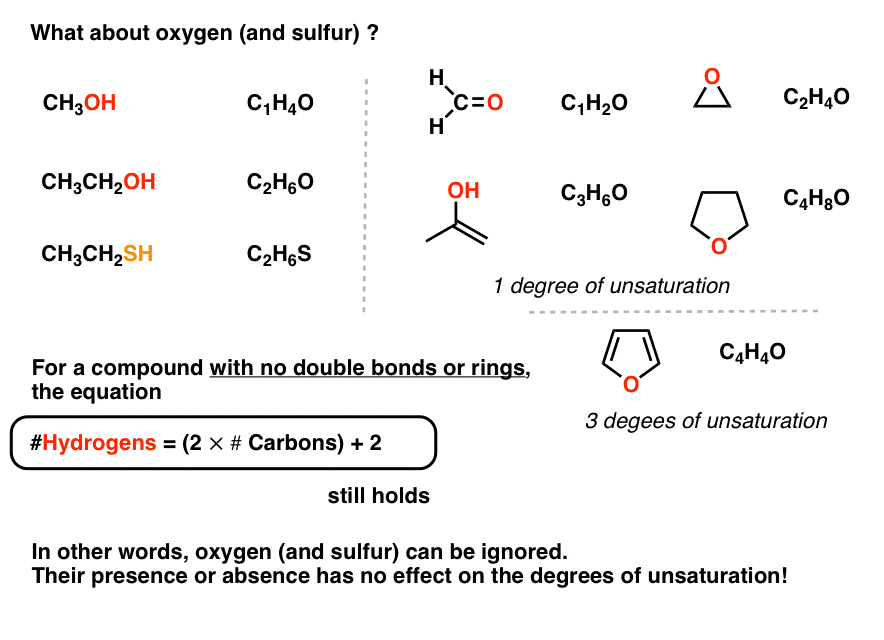
There are a few ways to do this. X1 - displaystyle fracyw-b2 Oxygen atoms dont contribute towards the degree of unsaturation of a compound. Any compound whose chemical formula has two hydrogens less than the maximum number possible 2n2 must have one ring or one double bond.
For example the Degrees of Unsaturation of tetrahydrofuran and cyclobutane are the same DU. 4 I - Iodine. In addition to this you should b.
The notion of the degree of unsaturation of an organic compound is derived simply from the tetravalency of carbon. Calculate the degree of unsaturation for benzene. Degrees of unsaturation is equal to 2 or half the number of hydrogens the molecule needs to be classified as saturated.
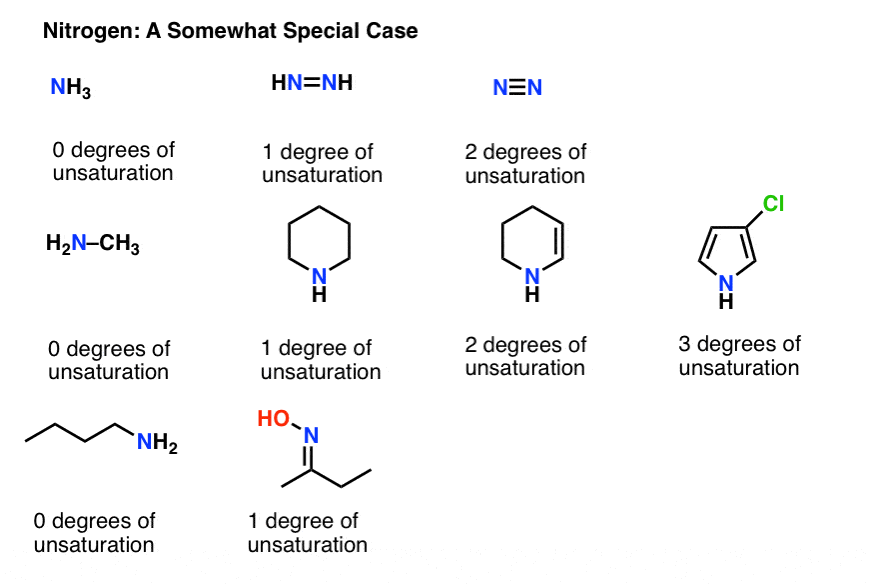
One for the double bond and one-half for the missing electron on nitrogen. 1 F - Fluorine. The notion of the degree of unsaturation of an organic compound is derived simply from the tetravalency of carbon.
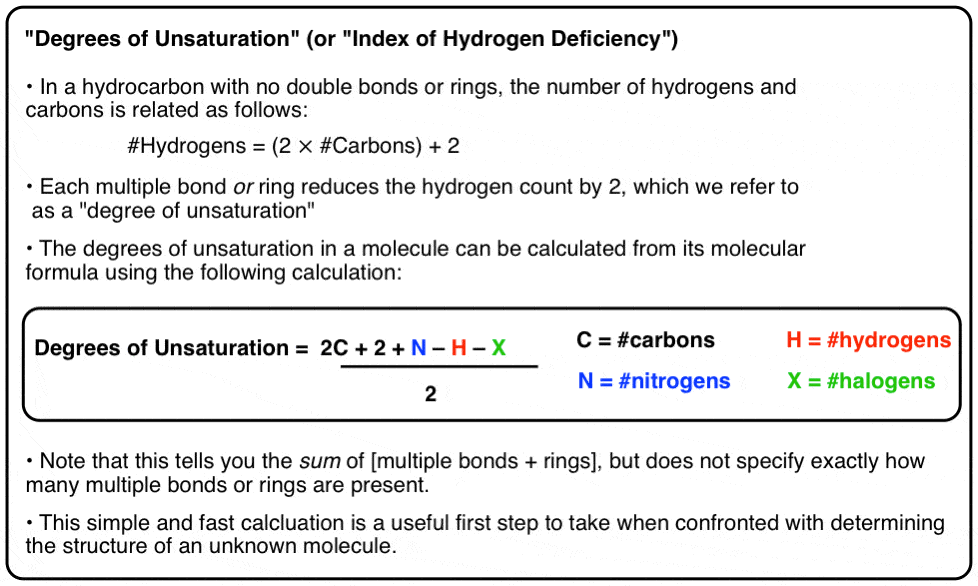
Hence the DoB formula divides by 2. Hence the DoB formula divides by 2. Assuming the compound to be C_xH_yO_zX_wN_b X represents halogens the D.
Answer 1 of 11. The formula subtracts the number of Xs because a halogen X replaces a hydrogen in a compound. The formula subtracts the number of Xs because a halogen X replaces a.
The Degree of Unsaturation is the rings plus the total number of multiple bonds. Any compound whose chemical formula has two hydrogens less than the maximum number possible 2n2 must have one ring or one double bond. Hence the DoB formula divides by 2.
This also has the effect of adding one degree of. Remove H s without changing the molecular weight. It is a remarkable fact however that regardless of which isomer you have drawn it must have the same degree of unsaturation as any other isomer -- molecular formula dictates the degree of unsaturation according to the formula below.
The Degree of Unsaturation is 7. Degrees of unsaturation is equal to 2 or half the number of hydrogens the molecule needs to be classified as saturated. Can you have a half degree of unsaturation.
However different elements can affect the formula used to calculate DoU. Given a particular hydrocarbon structure for which you know the number of carbons and the degree of unsaturation you can calculate the number of hydrogen atoms. The degree of unsaturation may also be employed in another way.
Degree of Unsaturation 2 2 x Carbons Nitrogens - Hydrogens - Halogens2 Any compound C 3 H 6 must have one degree of. The formula includes the number of hydrogen atoms added because of addition of nitrogen and subtracts the number of halogen atoms because they displace an equal number of. The formula subtracts the number of Xs because a halogen X replaces a hydrogen in a compound.
The degree of unsaturation is equal to 2 or half the total number of hydrogen that a molecule needs to be classified as saturated. Degrees of unsaturation is equal to 2 or half the number of hydrogens the molecule needs to be classified as saturated. You can use this elementary formula.
The formula would indicate that this molecule has 15 degrees of unsaturation. Now divide 14 by 2. Hence the DoB formula divides by 2.
3 Br - Bromine. Tap card to see definition. Degrees of unsaturation is equal to half the number of hydrogens the molecule needs to be fully saturated.
However I think it is mostly applied to organic compounds. Click again to see term. The formula is intended to tell you the total number of rings pi bonds which of course has to be a whole number.
Begingroup In principle for any negative charge there is half a degree of unsaturation. Tap again to see term. Then the answer is a qualified NO.
This has the effect of adding one degree of unsaturation. So a double negative charge is one degree of unsaturation. The compound needs 4 more hydrogens in order to be fully saturated expected number of hydrogens-observed number of hydrogens8-44.
As to the question as to why oxygen is dropped from the equation if the oxygen in hydroxychloroquine werent there the degree of unsaturation would still be the same. Click card to see definition. The compound needs 4 more hydrogens in order to be fully saturated expected number of hydrogens-observed number of hydrogens8-44.
The degree of unsaturation is equal to 2 or half the total number of hydrogen that a molecule needs to be classified as saturated. But the unsat formula isnt intended to handle all possible molecular formulas. Benzene is a cyclic compound with one ring.
1 Replace CH4 with O. Degrees of unsaturation is equal to 2 or half the number of hydrogens the molecule needs to be classified as saturated. 2 Cl - Chlorine.
Radicals will always have n-and-a-half degrees of unsaturation where n is any integer including zero. Hence the DoB formula divides by 2. Hence the degree of unsaturation formula divides the value by 2.
If youre referring to degrees of unsaturation as given by the formula. The DoU of compounds containing elements other than carbon and hydrogen can also be calculated in a similar fashion. This compound has 2 degrees of unsaturation 42 2.
2 Replace C2H4 with N2. Degrees of unsaturation is equal to 2 or half the number of hydrogens the molecule needs to be classified as saturated.
Degrees Of Unsaturation Or Ihd Index Of Hydrogen Deficiency

Degrees Of Unsaturation Or Ihd Index Of Hydrogen Deficiency
0 Response to "Can You Have a Half Degree of Unsaturation"
Post a Comment